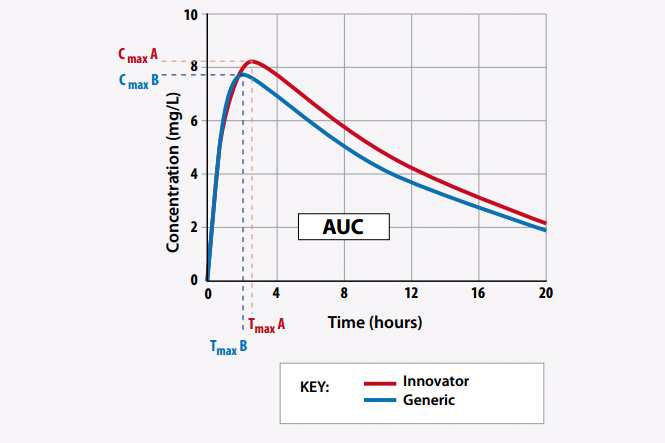
BIOEQUIVALENCE STUDY
The process of research and development of a new pharmaceutical is very time-consuming, extremely expensive. A new drug will be approved for market, this process can take 10 to 15 years and cost millions of dollars. Therefore, after researching a drug successfully, the manufacturer has drug patent and most drug patents are protected up to 20 years…


